Overview
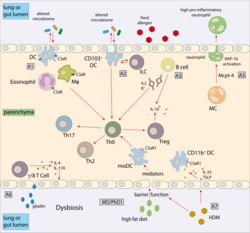
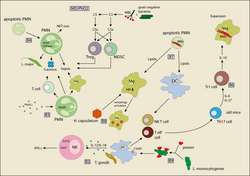
Innate and adaptive immune pathways are indispensable for the successful battle against pathogens, yet can cause a fatal outcome in allergic diseases by destroying cells and tissues. The medical, social and economic burden mediated by infection with intracellular pathogens and allergic diseases is enormous. One-third of the world's population is currently infected with Mtb. With regard to allergic diseases, the WHO estimates that 300 million people currently suffer from allergic asthma, which is the most common chronic disease among children. Thus, there is an urgent need for a better understanding of the immune mechanisms underlying such diseases as a prerequisite to define new therapeutic targets. Genetic approaches, including genome-wide association studies, have highlighted the complex nature of infectious and allergic diseases. Many of the identified genes or gene modifications relate to molecules of the immune system. The innate immune system recognizes pathogens by highly conserved sensor molecules that are either located in the fluid phase, at cell surfaces or within professional and non-professional immune cells. Although many pattern recognition systems have been identified during the past decade, a detailed understanding of how the immune system senses intracellular pathogens such as parasites (i.e. Toxoplasma gondii and Leishmania spp.), viruses or intracellular bacteria, including Mycobacterium tuberculosis, is still lacking. Importantly, several allergen components can also activate innate immune sensors by either mimicking parts of Toll-like receptor (TLR) 4, activating C-type lectin receptors, or G-Protein-coupled receptors, resulting in maladaptive Th2 deviation and the development of allergic disorders. Also, some allergens seem to mimic biochemical activities of parasite- associated proteases. Activation of innate immune sensors in a network of professional antigen-presenting cells (APC) such as DC and macrophages, as well as non-professional APCs including neutrophils, MCs and epithelial cells, instructs and activates T lymphocytes toward differentiation into effector or regulatory phenotypes. Appropriate differentiation and activation of different T lymphocyte subsets is required to successfully fight infections with intracellular pathogens; yet similar but undesired recognition of harmless exogenous, environmental molecules by innate immune sensors can drive maladaptive immune responses leading to allergic diseases. Despite the growing numbers of reports suggesting joint pathways of microbial and allergen sensing as well as regulation of adaptive or maladaptive immune responses following microbial and/or allergen contact, network approaches that link the two fields of infection and allergy are missing in Germany. This is surprising as a better understanding of such pathways may result in novel prophylactic and/or therapeutic concepts as indicated by the first successful clinical trials with parasitic worms as a new therapeutic option for allergy.
Please click here for more information the projects of the second generation and of the third generation of doctoral researchers.